Since May of this year, monkeypox cases have been reported in numerous parts of the world. According to a data update from the Centers for Disease Control and Prevention as of September 23, the number of human cases of the monkeypox virus in the US has reached 24,846.
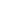
This disease is rare and caused by infection with the monkeypox virus (MPXV). This enveloped double-stranded DNA virus is part of the same family of viruses as the variola virus. It can be spread through contact with body fluids, skin lesions, or internal mucosal surfaces, and symptoms include fever, headache, muscle aches as key examples. The latent period of monkeypox generally ranges from 6 to 13 days but may also be shorter or longer 5 to 21 days (namely about three weeks).
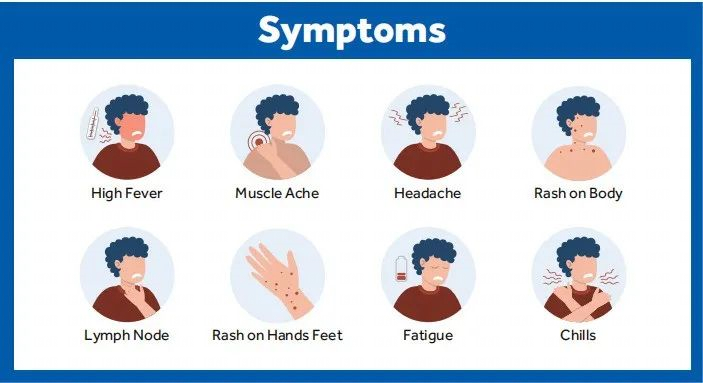
Although human infection with the monkeypox virus is less pathogenic than its cousin virus, the variola virus, it can still cause death, and there is still no effective therapy for the disease up to now. Therefore, we need to be more vigilant about the monkeypox virus. Only by proactively understanding how monkeypox spreads can we effectively avoid the risk of being infected.
Monkeypox is a zoonotic infectious disease and can spread from person to person. It spreads primarily through direct contact with the monkeypox rash, scabs, or body fluids from a person with monkeypox. It can also be spread through large respiratory droplets but requires prolonged face-to-face contact or intimate contact. Although a majority of the currently reported monkeypox cases were found in men who have sex with men, anyone in close contact with an infected person may also become infected.
Therefore, people should avoid close skin contact with anyone who has a rash like monkeypox, avoid touching items and materials used by any person with monkeypox, and wash hands frequently with soap and water or an alcohol-based hand sanitizer, especially before eating or touching the face and after using a bathroom.
To date, no specific therapy for monkeypox has been approved. But monkeypox and variola viruses are of similar genes, so vaccines developed to protect against variola can also be used to prevent monkeypox infection with about 85% effectiveness. In addition, speeding up the nucleic acid testing of the monkeypox virus is also significant for the prevention of the monkeypox virus.
The WHO stated that the best diagnostic specimens for monkeypox were obtained directly from rashes, fluids or crusts, or biopsies. Other methods, including antigen and antibody tests, may not work because they cannot distinguish orthopoxviruses that include the monkeypox virus.
A risk-oriented approach is recommended for use during the handling of the specimens from suspected, probable, or confirmed cases of monkeypox in laboratories. The testing program for the presence of the monkeypox virus is classified as hazard group 3 (HG3), so monkeypox virus specimens must be tested in BSL-2 or above laboratories with appropriate equipment.
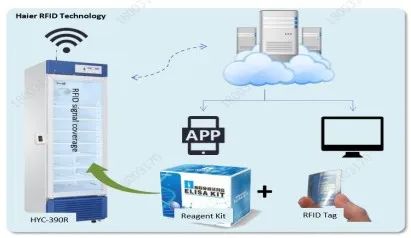
Laboratories need to follow the below procedures to test the monkeypox virus specimens upon receiving them. Let’s check how Haier Biomedical monkeypox solutions work for the entire process.
1.Specimen storage
Monkeypox virus specimens need to be refrigerated at 2°C to 8°C or frozen within one hour of collection (-20°C or lower). Such specimens shall be stored at -20°C or below if transported for more than 7 days. For long-term storage (more than 60 days upon collection), the specimen needs to be preserved at -70°C or below.

Haier Biomedical’s laboratory refrigerators, laboratory freezers, and ULT freezers are designed with HC refrigeration systems and microprocessors for accurate temperature control. For example, the HYC series laboratory refrigerators can maintain the inner temperature between 2 and 8 ℃; the DW-86 series ULT freezers keep the temperature at -86℃, which is ideal for storing temperature-sensitive biological samples requiring critical storage conditions.
2.Specimen processing and preparation
The Monkeypox virus may spread during the specimen processing phase if any material becomes contaminated or improper testing methods are used. Therefore, biosafety measures must be enhanced, and specimens from patients with suspected monkeypox virus infection must be prepared in level-2 biosafety cabinets before inactivation.


In addition to the protection provided by biosafety cabinets, Haier Biomedical’s level-2 biosafety cabinets can also be coated with antibacterial powder on all exterior and interior painted surfaces for increased safety. Centrifuges are also necessary for separating liquid and solid components of specimens, and in this case, safety cups or canned rotors are recommended for use.
3.Testing and analysis
A nucleic acid amplification test (NAAT) needs to be performed to confirm MPXV infection. For the detection of unique viral DNA sequences, either real-time PCR or conventional polymerase chain reaction (PCR) can be used. Virus detection generally involves two steps. The first PCR detects orthopoxvirus but does not identify the species, whereas the second PCR (or sequencing) tests MPXV specifically.


Haier Biomedical’s PCR thermal cycler enables efficient nucleic acid amplification for virus analysis. The product also supports independently operated PCR program input, monitoring, and analysis.
Committed to the development of global healthcare, Haier Biomedical strives to deliver comprehensive scenario solutions worldwide, including biobank, pharmaceutical, R&D, hospital, laboratory to name but a few, to solve their different problems and lead the booming development of the biomedical and life science industry.












.png)